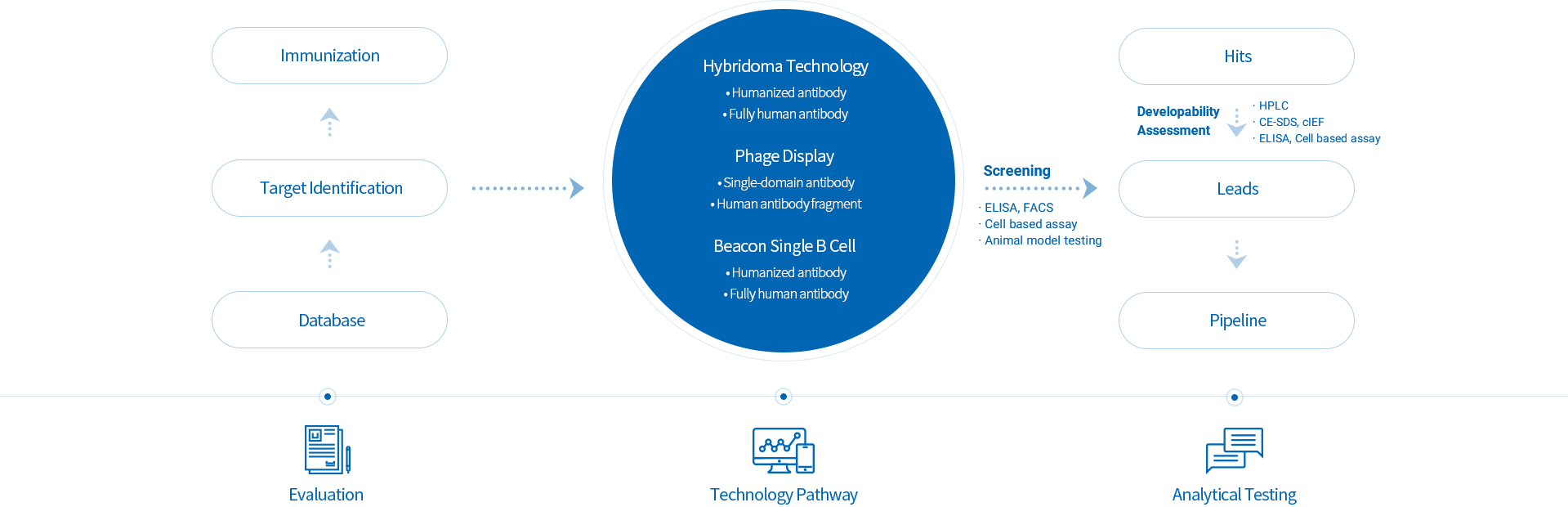
Our in-house systemic platform covers from the discovery to the start to the end, including commercial manufacturing


Analysis and Evaluation Platform
The platform is equipped with state-of-the-art instruments, such as high-resolution mass spectrometers, flow cytometer, Fortebio, capillary electrophoresis, ultra-high performance liquid chromatograph, iCE3, qPCR instrument, plate reader, etc. It also has a variety of reporter gene cell lines that facilitate the functional assay evaluation, such as ADCC, ADCP, etc. The platform has comprehensive analytical technologies to support IND/BLA filing, including drug developability assessment, antibody Fc engineering, analytical method development and validation, releasing and stability testing, clinical stability studies, structure characterization, potency and epitope studies, degradation pathway studies, CQA establishment, and quality comparability study.

Process Development Platform
The platform is furnished with a variety of advanced instruments, including Cytena c.sight, Sartorius 24×Ambr15, Thermo G3Lab 16×3L BRs, and AKTA Pure M& Avant protein chromatography system, etc., which can meet the process development needs from cell line construction, upstream cell culture, protein purification to formulation filling. Meanwhile, the platform has a highly efficient CHO-K1 expression system, which has been validated in multiple mAbs, bi-specific and Sd-Fc antibodies. With the highest 9.2 g/L, most production cell line expression levels reach 4-7 g/L and the IND filing can be achieved within 15 months.

GMP Manufacturing Platform
The platform is equipped with Cytiva, Sartiorus and other advanced bioreactors of famous brand, protein chromatography system and filling system, etc., for mammal cell culture, protein purification to formulation filling; Manufacturing facilities meet the requirements of Good Manufacturing Practice (GMP) , FDA, NMPA and EMA. The pilot capacity is 3 × 200L and 1 × 500L disposable bioreactors, which can flexibly provide qualified samples from preclinical stage to Phase I and Phase II clinical.

Quality Management Platform
The platform adheres to our quality policy of "innovation, ingenuity, quality-oriented and sincerity”, and follows regulations of health authorities in different countries (CN, EU, and US), covering through the total four stages of the product life cycle: drug development, technology transfer, manufacturing, withdrawn(termination). We adhere to the concept of quality from design, and integrate quality management into products, processes and analytical testing methods, to make sure products meet the quality standards and ensure safety and efficacy.

Regulatory Affairs Platform
The platform provides regulatory support from the start to the end, including regulatory affairs strategy, application submission, communication with health authorities and preparing filling materials. Our regulatory team has a deep understanding of guidelines of NMPA, FDA, and EMA, and keep tracking updated guidelines. Our strict summarizing ability of scientific data and rigorous review ability can solve the issues of regulatory affairs efficiently.
Our immune checkpoints include multiple anti-tumor pathways through the tumor microenvironment. The bolded mechanisms and their corresponding targets indicate drugs that have started preclinical or clinical investigations by Dragon Boat, while the others are currently in early development.
Immune suppression: PD-L1, CSF1R, CD47
Anti-tumor response: EGFR, CLDN18.2
Suppressor recruitment: CD73
Angiogenesis: PAUF
Epithelial-Mesenchymal Transition(EMT)
ECM Remodeling
Chronic Inflammation
Immunogenic Cell Death
Hypoxia
Starting with traditional monoclonal antibodies, Dragonboat gradually expanded modalities to Sd-Fc, BsAband TsAb, in order to bring unlimited possibilities to immunotherapy
EGFR
PD-L1
IL-4R
CSF-1R

EGFR
PD-L1
IL-4R
CSF-1R

EGFR
PD-L1
IL-4R
CSF-1R

EGFR
PD-L1
IL-4R
CSF-1R
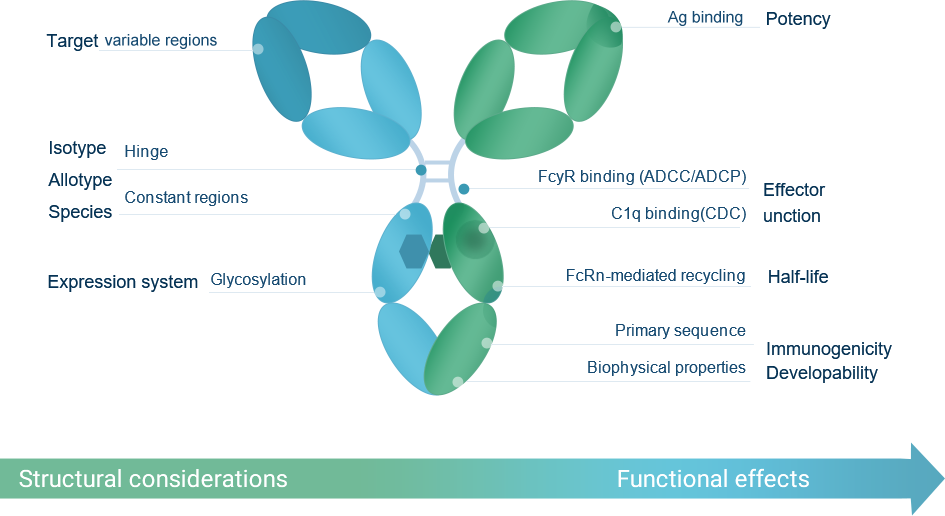
Dragonboat accumulated much experience in Fc engineeringBased on different targets and MoA, we select the appropriate structure and subtype and conduct engineering accordingly